H3K27ac(組蛋白H3賴氨酸27乙?��;?抗體符合EpiCypher的批次特異性SNAP-Certified?標(biāo)準(zhǔn)��,在CUT&RUN和CUT&Tag應(yīng)用中具有特異性和高效的靶標(biāo)富集����。這需要與相關(guān)組蛋白 PTMs (使用加標(biāo)對照的 SNAP-CUTANA? K-AcylStat Panel 測定���,EpiCypher RD193002)的交叉反應(yīng)性 <20%(圖 1 和 圖5)�����。在不同的細(xì)胞起始條件下��,一致的基因組富集結(jié)果證實了高靶標(biāo)效率:CUT&RUN中500k和50k的細(xì)胞量(圖2-3)����,CUT&Tag中100k和10k的細(xì)胞量(圖6-7)。即使在細(xì)胞數(shù)量減少的情況下��,高效抗體也顯示出相似的峰結(jié)構(gòu)(圖3和7)和高度保守的全基因組信號(圖2和6)�����。H3K27ac與基因激活相關(guān)��,并在活性增強(qiáng)子和啟動子中富集[1]�����。
產(chǎn)品詳情
產(chǎn)品名稱:H3K27ac Antibody, SNAP-Certified? for CUT&RUN and CUT&Tag
宿主來源:Rabbit
實驗應(yīng)用:CUT&RUN, CUT&Tag
免疫原:A synthetic peptide corresponding to histone H3 acetylated at lysine 27
克隆性:Monoclonal[2114-3E4]
保存溫度:自收到之日起�����,4℃下可穩(wěn)定儲存1年����。
驗證數(shù)據(jù)
—— CUT&RUN
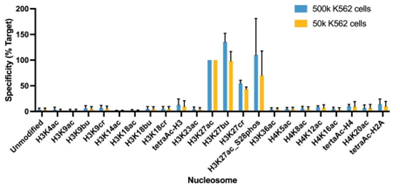
Figure 1: Average SNAP specificity analysis from two CUT&RUN experiments |
CUT&RUN was performed as described in Figure 4. CUT&RUN sequencing reads were aligned to the unique DNA barcodes corresponding to each nucleosome in the K-AcylStat panel (x-axis). Data are expressed as a percent relative to on-target recovery (H3K27ac set to 100%). The antibody showed recovery of H3K27ac spike-in nucleosomes as well as H3K27ac nucleosomes that contain a proximal phosphorylation at S28 at both 500k and 50k cells. The antibody cross-reacts with extended acyl states (butyrylation and crotonylation) at H3K27, but these are typically low abundance in cells?[2].
|
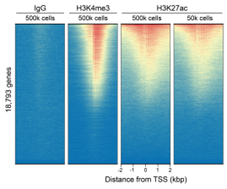
Figure 2: CUT&RUN genome-wide enrichment |
CUT&RUN was performed as described in?Figure 4. Sequence reads were aligned to 18,793 annotated transcription start sites (TSSs ± 2 kbp). Signal enrichment was sorted from highest to lowest (top to bottom) relative to the H3K27ac - 500k cells reaction (all gene rows aligned). High, medium, and low intensity are shown in red, yellow, and blue, respectively. H3K4me3 positive control and H3K27ac antibodies produced the expected enrichment pattern, which was consistent between 500k and 50k cells and greater than the IgG negative control.
|
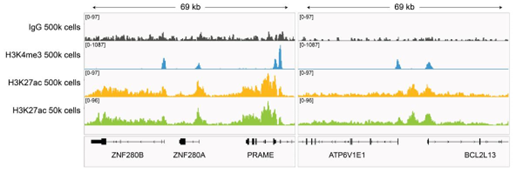
Figure 3: H3K27ac CUT&RUN representative browser tracks |
CUT&RUN was performed as described in?Figure 4. Gene browser shots were generated using the Integrative Genomics Viewer (IGV, Broad Institute). H3K27ac antibody tracks display peaks at promoters and enhancers, consistent with the biological function of this PTM. Similar results in peak structure and location were observed for both 500k and 50k cell inputs.
|
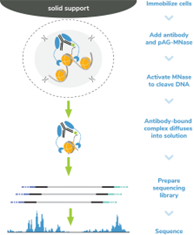
Figure 4: CUT&RUN methods |
CUT&RUN was performed on 500k and 50k K562 cells with the SNAP-CUTANA? K-MetStat Panel (EpiCypher?19-1002) or SNAP-CUTANA? K-AcylStat Panel (EpiCypher RD193002) spiked-in prior to the addition of 0.5 μg of either IgG negative control (EpiCypher?13-0042), H3K4me3 positive control (EpiCypher?13-0041), or H3K27ac antibodies. The experiment was performed using the CUTANA? ChIC/CUT&RUN Kit v3 (EpiCypher?14-1048). Library preparation was performed with 5 ng of CUT&RUN enriched DNA (or the total amount recovered if less than 5 ng) using the CUTANA? CUT&RUN Library Prep Kit (EpiCypher?14-1001/14-1002). Both kit protocols were adapted for high throughput Tecan liquid handling. Libraries were run on an Illumina NextSeq2000 with paired-end sequencing (2x50 bp). Sample sequencing depth was 3.8 million reads (IgG 500k cell input), 5.0 million reads (IgG 50k cell input), 2.5 million reads (H3K4me3 500k cell input), 9.1 million reads (H3K4me3 50k cell input), 12.6 million reads (H3K27ac 500k cell input), and 11.0 million reads (H3K27ac 50k cell input). Data were aligned to the hg19 genome using Bowtie2. Data were filtered to remove duplicates, multi-aligned reads, and ENCODE DAC Exclusion List regions.
|
——?CUT&Tag
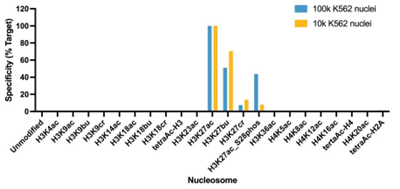
Figure 5: SNAP specificity analysis in CUT&Tag |
CUT&Tag was performed as described in?Figure 8. CUT&Tag sequencing reads were aligned to the unique DNA barcodes corresponding to each nucleosome in the K-AcylStat panel (x-axis). Data are expressed as a percent relative to on-target recovery (H3K27ac set to 100%). The antibody showed recovery of H3K27ac spike-in nucleosomes and to a lesser extent H3K27ac nucleosomes that contain a proximal phosphorylation at S28 at both 500k and 50k cells. The antibody cross-reacts with butyrylation at H3K27, but this is typically low abundance in cells [2].
|

Figure 6: CUT&Tag genome-wide enrichment |
CUT&Tag was performed as described in?Figure 8. Sequence reads were aligned to 18,793 annotated transcription start sites (TSSs ± 2 kbp). Signal enrichment was sorted from highest to lowest (top to bottom) relative to the H3K27ac - 100k nuclei reaction (all gene rows aligned). High, medium, and low intensity are shown in red, yellow, and blue, respectively. H3K4me3 positive control and H3K27ac antibodies produced the expected enrichment pattern, which was consistent between 100k and 10k nuclei and greater than the IgG negative control.
|
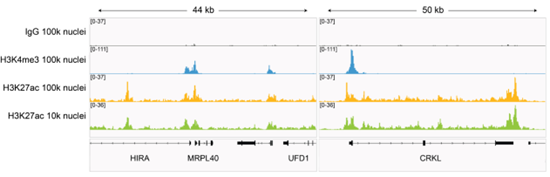
Figure 7: H3K27ac CUT&Tag representative browser tracks |
CUT&Tag was performed as described in?Figure 8. Gene browser shots were generated using the Integrative Genomics Viewer (IGV, Broad Institute). H3K27ac antibody tracks display peaks at promoters and enhancers, consistent with the biological function of this PTM. Similar results in peak structure and location were observed for both 100k and 10k nuclei inputs.
|
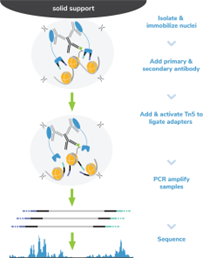
Figure 8: CUT&Tag methods |
CUT&Tag was performed on 100k and 10k K562 nuclei with the SNAP-CUTANA? K-MetStat Panel (EpiCypher?19-1002) or SNAP-CUTANA? K-AcylStat Panel (EpiCypher RD193002) spiked-in prior to the addition of 0.5 μg of either IgG negative control (EpiCypher?13-0042), H3K4me3 positive control (EpiCypher?13-0041), or H3K27ac antibodies. The experiment was performed using the CUTANA? CUT&Tag Kit v1 (EpiCypher?14-1102/14-1103). Libraries were run on an Illumina NextSeq2000 with paired-end sequencing (2x50 bp). Sample sequencing depth was 1.3 million reads (IgG 100k nuclei input), 2.0 million reads (IgG 10k nuclei input), 3.5 million reads (H3K4me3 100k nuclei input), 8.0 million reads (H3K4me3 10k nuclei input), 8.1 million reads (H3K27ac 100k nuclei input) and 8.5 million reads (H3K27ac 10k nuclei input). Data were aligned to the hg19 genome using Bowtie2. Data were filtered to remove duplicates, multi-aligned reads, and ENCODE DAC Exclusion List regions.
|
?
訂購詳情
貨號 | 產(chǎn)品名稱 | 規(guī)格 |
13-0059 | H3K27ac Antibody, SNAP-Certified? for CUT&RUN and CUT&Tag | 100 μg |
參考文獻(xiàn)
[1] Pei et al. Clinical Epigenetics (2020). PMID: 32664951
[2] Simithy et al. Nature Communications (2017). PMID: 29070843
?

如需了解更多詳細(xì)信息或相關(guān)產(chǎn)品�����,
請聯(lián)系EpiCypher中國授權(quán)代理商-欣博盛生物?
全國服務(wù)熱線: 4006-800-892 ? ? ??郵箱: market@neobioscience.com?
深圳: 0755-26755892 ? ? ? ??北京: 010-88594029 ???????????
廣州:020-87615159????????? ?上海: 021-34613729
代理品牌網(wǎng)站: m.yuebanme.com?
自主品牌網(wǎng)站: www.neobioscience.ne